Neuronal ER-plasma membrane junctions couple excitation to Ca2+-activated PKA signaling
Nature Communications volume 14, Article number: 5231 (2023)
内质网 (ER) 和质膜 (PM) 之间的连接是真核细胞中普遍存在的特殊膜接触。 细胞内信号传导机制在 ER-PM 连接附近的集中使得这些结构域在脂质和 Ca2+ 信号传导和稳态中发挥关键作用。 蛋白激酶 A (PKA) 信号传导的亚细胞区室化也调节重要的细胞功能,然而,PKA 和 ER-PM 连接域之间的具体关联尚不清楚。 在这里,我们发现在大脑神经元中,I 型 PKA 通过 SPHKAP(一种 I 型 PKA 特异性锚定蛋白)定向到 Kv2.1 通道依赖性 ER-PM 连接域。 SPHKAP 与 I 型 PKA 调节亚基 RI 和 ER 驻留 VAP 蛋白的关联导致 I 型 PKA 在与 ER-PM 连接相关的堆叠 ER 池之间集中。 这种与 ER 相关的 PKA 信号体能够实现 PKA 和 Ca2+ 信号机制之间的相互调节,以支持 Ca2+ 流入和兴奋转录耦合。 这些数据表明,神经元 ER-PM 连接支持由膜去极化和细胞内 Ca2+ 驱动的不依赖于受体的 PKA 信号传导,从而可以将电信号中编码的信息转换为整个细胞普遍识别的生化变化。
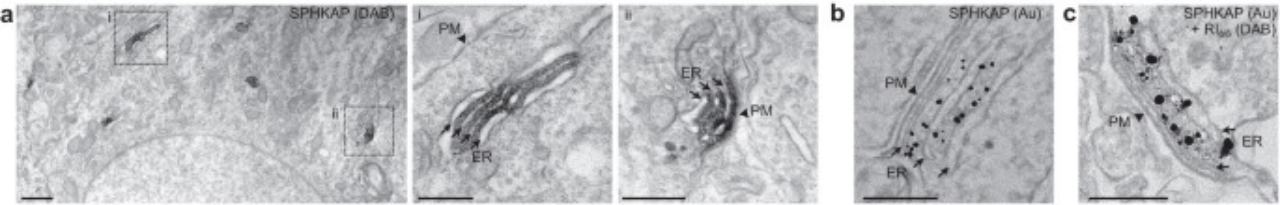
a EM images of SPHKAP-immunoperoxidase DAB reaction product acquired from the soma of a CA1 pyramidal neuron in a rat brain section. High-magnification images of insets are provided on right. Scale bars, 1 µm (left panel) and 500 nm (insets i & ii). Images are representative of results obtained from n = 2 rats.
b EM image of SPHKAP-immunogold particles acquired from the soma of a CA1 pyramidal neuron in a mouse brain section. Scale bar, 200 nm. Images are representative of results obtained from n = 2 mice.
c EM image of SPHKAP-immunogold particles and RI-immunoperoxidase reaction product acquired from the soma of a CA1 pyramidal neuron in a mouse brain section. Scale bar, 200 nm. Images are representative of results obtained from n = 2 mice.
a 从大鼠脑切片的 CA1 锥体神经元胞体获取的 SPHKAP-免疫过氧化物酶 DAB 反应产物的电镜图像。 右侧提供了插图的高倍放大图像。 比例尺,1μm(左图)和 500nm(插图 i 和 ii)。 图像代表从 n = 2 只大鼠获得的结果。
b 从小鼠大脑切片的 CA1 锥体神经元胞体获取的 SPHKAP-免疫金颗粒的电镜图像。 比例尺,200 nm。 图像代表从n = 2只小鼠获得的结果。
c 从小鼠大脑切片的 CA1 锥体神经元胞体获取的 SPHKAP-免疫金颗粒和 RI-免疫过氧化物酶反应产物的 EM 图像。 比例尺,200 nm。 图像代表从n = 2只小鼠获得的结果。
Immunogold-EM
Pre-embedding immunogold electron microscopy was performed as described previously62. All mouse handling and sample preparation for pre-embedding immunoelectron microscopy was performed at the Institute of Science and Technology Austria and were conducted under the license approved by the Austrian Federal Ministry of Science and Research and the Austrian and EU animal laws. Deeply anesthetized mice were transcardially perfused with 25 mM PBS, pH 7.4, for 1 min, followed by a fixative containing 4% formaldehyde (freshly prepared from paraformaldehyde), 0.05% glutaraldehyde, and 15% saturated picric acid made up in 0.1 M phosphate buffer (PB), pH 7.4, for 12 min. After perfusion, brains were quickly removed from the skull, washed briefly with PB, and coronally sectioned at 50 μm with a microslicer (Pro-7 linear slicer, Dosaka). Sections were rinsed several times in PB, freeze-thawed, quenched with 50 mM glycine in Tris-buffered saline (TBS) pH 7.4 at room temperature for 1 h, blocked in 10% NGS and 2% bovine serum albumin in TBS for 1 h, and then incubated in primary antibodies made up in TBS containing 1% NGS overnight at 4 °C. For single labeling for SHPKAP, mouse monoclonal anti-SPHKAP IgG1 antibody L131/17 (at 2 μg/mL) was used. For the double labeling, the SHPKAP antibody was combined with rabbit antibodies for PKARI (1 μg/mL, Cell Signaling Technology). After washing, the sections were incubated with 1.4 nm gold-coupled anti-mouse secondary antibody (Nanoprobes Cat# 2001) diluted in TBS at a ratio of 1:100. After washing, the sections were postfixed in 1% glutaraldehyde for 10 min, followed by silver enhancement of the immunogold particles using a HQ silver EM intensification kit (Nanoprobes Cat# #2012). Sections for the double labeling were washed in TBS and incubated with biotinylated goat anti-rabbit IgG antibody (1:200, Vector Labs Cat# BA-1000) at room temperature overnight. The sections were washed in TBS and then reacted with an avidin-biotinylated horseradish peroxidase complex (1:100 Vectastain Elite, Vector Labs Cat# PK-6200) made up in TBS for 2 h. After three washes in TBS and one in 50 mm Tris-HCl buffer (TB), pH 7.4, the peroxidase activity was visualized by incubating sections in TB containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (Dojindo Molecular Technologies) and 0.003% hydrogen peroxide. Sections for both single and double labeling were then postfixed with 1% osmium tetroxide for 20 min, en bloc counterstained with 1% uranyl acetate for 30 min, and dehydrated in graded ethanol series followed by propylene oxide. The sections were infiltrated overnight at room temperature in Durcapan resin (Sigma-Aldrich Cat# 44611) and transferred to glass slides for flat embedding. After resin curing at 60 °C for 48 h, the trimmed tissues from the CA1 area of the hippocampus were re-embedded in Durcapan resin blocks for ultrathin sectioning. Serial 70-nm-thick sections were cut from the surface (within 3 μm depth) of the samples and were collected on pioloform-coated single-slot copper grids. Images were captured with a CCD camera (Veleta, Olympus SIS) connected to a Tecnai 12 transmission electron microscope (FEI).
免疫金-EM
如前所述进行包埋前免疫金电子显微镜观察。 包埋前免疫电子显微镜的所有小鼠处理和样品制备均在奥地利科学技术研究所进行,并在奥地利联邦科学与研究部以及奥地利和欧盟动物法批准的许可下进行。 深度麻醉的小鼠经心灌注 25 mM PBS(pH 7.4)1 分钟,然后用 0.1 M 磷酸盐缓冲液配制的含有 4% 甲醛(由多聚甲醛新鲜制备)、0.05% 戊二醛和 15% 饱和苦味酸的固定剂灌注 (PB),pH 7.4,12 分钟。 灌注后,将大脑快速从头骨中取出,用PB简单清洗,并用微切片机(Pro-7线性切片机,Dosaka)进行50μm的冠状切片。 将切片在 PB 中冲洗几次,冻融,用 Tris 缓冲盐水 (TBS) pH 7.4 中的 50 mM 甘氨酸在室温下淬灭 1 小时,在 TBS 中的 10% NGS 和 2% 牛血清白蛋白中封闭 1 小时 ,然后在含有 1% NGS 的 TBS 中配制的一抗在 4°C 下孵育过夜。 对于 SHPKAP 的单一标记,使用小鼠单克隆抗 SPHKAP IgG1 抗体 L131/17(2μg/mL)。 对于双重标记,SHPKAP 抗体与 PKARI 兔抗体(1μg/mL,Cell Signaling Technology)结合。 清洗后,将切片与以 1:100 的比例稀释于 TBS 中的 1.4 nm 小鼠二抗 (Nanoprobes Cat# 2001) 一起孵育。 清洗后,将切片在 1% 戊二醛中后固定 10 分钟,然后使用 HQ 银 EM 增强试剂盒(Nanoprobes Cat# #2012)对免疫金颗粒进行银增强。 双标记切片在 TBS 中洗涤,并与生物素化山羊抗兔 IgG 抗体(1:200,Vector Labs Cat# BA-1000)在室温下孵育过夜。 将切片在 TBS 中洗涤,然后与在 TBS 中配制的抗生物素蛋白-生物素化辣根过氧化物酶复合物(1:100 Vectastain Elite,Vector Labs Cat# PK-6200)反应 2 小时。 在 TBS 中洗涤 3 次,在 50 mm Tris-HCl 缓冲液 (TB)(pH 7.4)中洗涤 1 次后,通过在含有 0.025% 3,3'-二氨基联苯胺四盐酸盐(Dojindo Molecular Technologies)和 0.003% 氢气的 TB 中孵育切片来观察过氧化物酶活性。 过氧化物。 然后将单标记和双标记的切片用 1% 四氧化锇后固定 20 分钟,用 1% 乙酸双氧铀整体复染 30 分钟,并在梯度乙醇系列中脱水,然后用环氧丙烷脱水。 将切片在室温下在 Durcapan 树脂(Sigma-Aldrich Cat# 44611)中渗透过夜,并转移至载玻片上进行平面包埋。 树脂在60℃固化48小时后,将海马CA1区修剪后的组织重新包埋在Durcapan树脂块中进行超薄切片。 从样品表面(深度在 3μm 以内)切下连续 70 nm 厚的切片,并收集在 pioloform 涂层的单槽铜网上。 使用连接到 Tecnai 12 透射电子显微镜 (FEI) 的 CCD 相机(Veleta、Olympus SIS)捕获图像。